|
Treatment is ‘promising option,’ more studies needed, researchers say by Margarida Maia, PhD | May 14, 2024 Local injection with botulinum toxin type A (BTX-A), such as Botox, may ease the severity of pain associated with Raynaud’s phenomenon in people with scleroderma, also known as systemic sclerosis, a meta-analysis study found.
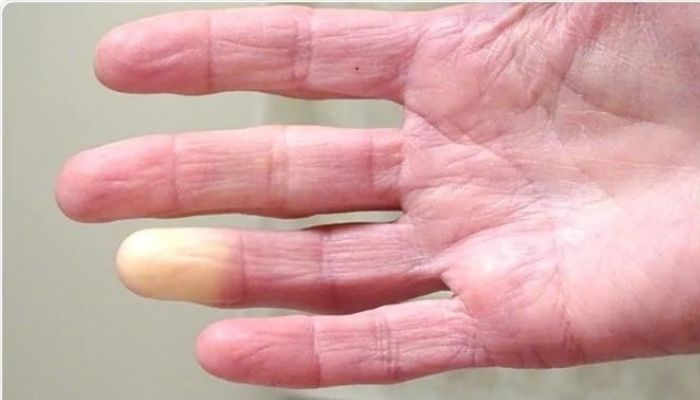
The study, “Efficacy and safety of botulinum toxin in treating scleroderma-associated raynaud’s phenomenon: a systematic review and meta-analysis,” was published as a research letter in the Archives of Dermatological Research. Scleroderma affects the connective tissue that holds tissues and organs together. While it mainly causes the skin to harden, scleroderma also can damage internal organs and blood vessels. It often starts with Raynaud’s phenomenon, which may appear long before other symptoms. Raynaud’s phenomenon limits blood flow to fingers and toes due to cold or stress. As a result, fingers and toes can change color and become numb or prickly. This is often accompanied by pain and sometimes digital ulcers or sores. Bacteria-derived BTX-A, often injected locally to smooth wrinkles on the face, among other uses, is being tested as a treatment for Raynaud’s phenomenon secondary to scleroderma. It’s thought that it may work in part by widening the blood vessels and reducing pain. Almost 20% of people in study have low red blood cells or low hemoglobin by Patricia Inácio, PhD | May 7, 2024 Anemia is found in almost 20% of people with systemic sclerosis (SSc) and is associated with an almost two times higher risk of death, a French study reports.
Iron deficiency was the most common reason for anemia in SSc, with older patients and those with diffuse cutaneous SSc (dcSSc) more likely to have anemia, which is a low number of red blood cells or low levels of hemoglobin. The study, “Prevalence, causes, and clinical associations of anemia in patients with systemic sclerosis: A cohort study,” was published in the Journal of Scleroderma and Related Disorders. Also called scleroderma, SSc typically leads to scarring in the skin due to the excessive production of collagen, the main protein component of scar tissue. Scarring can also affect internal organs, including the lungs, heart, kidney, and digestive tract. Anemia is considered a risk factor for more severe outcomes in SSc. Here, a team led by researchers in France analyzed data from 502 SSc patients (mean age, 58; 85.7% women), diagnosed at the Lille University Hospital to learn more about how frequently anemia occurs and the underlying causes of it in SSc. Most of the patients (77.5%) had limited SSc and 21.6% had dcSSc. Formula could ID patients in need of 'enhanced managing strategies,' study says by Lindsey Shapiro, PhD | April 30, 2024 Researchers have developed a model for predicting which systemic sclerosis (SSc) patients with interstitial lung disease (ILD) — together known as SSc-ILD — will experience progressive lung function declines that are known to be associated with a poorer prognosis.
The prediction algorithm accounts for nine clinical factors, including age, certain SSc features and symptoms, therapy use, and disease-associated antibodies. All were found to be linked to the development of this aggressive form of ILD — called progressive fibrosing interstitial lung disease or PF-ILD — in a group of about 300 patients. “This study developed the first prediction model for PF-ILD in patients with SSc-ILD,” the researchers wrote, noting that “the established formula … could help in identifying patients with the highest risk, who should receive enhanced managing strategies during follow-up.” Titled “Prediction of progressive fibrosing interstitial lung disease in patients with systemic sclerosis: insight from the CRDC cohort study,” the work was published in RMD Open. Half of patients in study found to have aggressive disease type PF-ILD In SSc, also called systemic scleroderma, an overactive immune system drives inflammation and scar tissue buildup — known as fibrosis — in the skin and internal organs. When the lungs are affected, patients may experience ILD, then named SSc-ILD, where the lung tissue is scarred and damaged, and breathing becomes more difficult. The severity and progression of interstitial lung disease can vary. PF-ILD, a progressive form, is marked by “rapidly aggravating [shortness of breath], progressive deteriorating lung function, declining physical functional capacity, worsening health-related quality of life, adverse therapeutic response and often early mortality,” the researchers wrote. An earlier identification of which patients are likely to experience this type of progression may enable a sooner start to interventions that could slow it down or stop it, preventing irreversible lung damage, according to the team. While some studies have reported a number of potential predictive factors of PF-ILD, an accurate model for identifying SSc patients who might be at a high risk of the aggressive lung disease has yet to be developed. Now, a team of scientists in China sought to develop one such model by looking at clinical information from 304 SSc-ILD patients who were registered in a Chinese database. Meta-analysis has implications for prognosis and prevention, researchers sayby Andrea Lobo, PhD | April 23, 2024 Having scleroderma increases the risk of developing diseases affecting blood vessels of the brain or heart, known as cerebrovascular and cardiovascular diseases, a review study found.
Relative to controls, the risk of stroke — caused by poor blood flow to the brain — in scleroderma patients was 64% higher, whereas that of cardiovascular disease was 112% higher. “Our findings support increased clinical surveillance and consideration of preventative strategies in this high-risk population,” researchers in Taiwan wrote. Their study, “Association between systemic sclerosis and risk of cerebrovascular and cardiovascular disease: a meta-analysis,” was published in Scientific Reports. Scleroderma, also known as systemic sclerosis (SSc), is characterized by the excessive production of collagen that leads to the accumulation of scar tissue, affecting the skin and potentially internal organs, such as the digestive tract and lungs. The disease may also damage blood vessels, both small and large, leading to complications such as pulmonary arterial hypertension and scleroderma renal crisis. Evidence has suggested that people with scleroderma may have an increased risk of stroke, heart attack, and peripheral vascular disease, which is reduced circulation in blood vessels outside the brain and heart. However, “the risk of cerebrovascular and cardiovascular complications attributed to SSc remains debated,” the researchers wrote. Developer says therapy has potential to offer 'first-in-class' benefits by Andrea Lobo, PhD | April 16, 2024 The U.S. Food and Drug Administration (FDA) has granted orphan drug status to BLR-200, a medication that BLR Bio — a company stemming from the Helix 51 biomedical incubator at Rosalind Franklin University — is developing for scleroderma.
According to Ronald Kaplan, PhD, the university’s executive vice president for research, “BLR-200 has the potential to establish first-in-class clinical benefits by addressing multiple elements driving scleroderma and treating multiple organs negatively impacted by the disease.” Orphan drug status is a designation given by the FDA to investigational therapies for rare diseases — those affecting less than 200,000 people in the U.S. It provides incentives such as exemptions from certain FDA fees, tax credits for clinical costs, and seven years of market exclusivity if the drug is ultimately approved. It’s an important milestone for BLR Bio’s drug candidate, Kaplan said in a university press release, which stated that “BLR-200 is different from other therapies now in development for the treatment of systemic sclerosis.” |
AuthorScleroderma Queensland Support Group Archives
July 2024
Categories
All
|
Scleroderma Association of Queensland
©Scleroderma Association of Queensland. All rights reserved. Website by Grey and Grey.