|
SCOT trial studied potential benefits over high-dose Cytoxan by Andrea Lobo, PhD | July 16, 2024 Patients with systemic sclerosis (SSc) who have a stem cell transplant see normalized gene activity related to immune function up to 4.5 years after the procedure.
That’s according to recent data from samples in the Phase 2/3 SCOT trial (NCT00114530), wherein the potential benefits of a stem cell transplant were compared with those of high-dose Cytoxan (cyclophosphamide) in severe SSc. “The normalization of the SSc [molecular] signature … parallels the clinical benefits of [stem cell transplant] at this time point and provides support for these disease-related pathways as therapeutic targets,” the researchers wrote in “Myeloablation Followed by Hematopoietic Stem Cell Transplantation and Long-Term Normalization of Systemic Sclerosis Molecular Signatures,” which was published in Arthritis & Rheumatology. SSc, or scleroderma, is an autoimmune disease where scar tissue (fibrosis) accumulates in the skin and possibly in the organs, such as the lungs, heart, or kidneys. Transplanting hematopoietic stem cells (HSC), which are precursors to all types of blood cells, has been investigated as a way to reset the immune system in people with SSc. Ferroptosis, natural block on uncontrolled cell growth, seen to be suppressed by Lindsey Shapiro, PhD | July 9, 2024 Ferroptosis, a cell death process that can help to regulate uncontrolled cell growth, was suppressed in skin cells from people with systemic sclerosis (SSc), according to recent research.
Such suppression appears to be mediated by increased activity of an antioxidant protein called GPX4. Researchers believe that targeting GPX4 to increase ferroptosis could offer a way of preventing the uncontrolled cell growth that drives scar tissue buildup, or fibrosis, in the skin of SSc patients. The study, “Upregulation of GPX4 drives ferroptosis resistance in scleroderma skin fibroblasts,” was published in Free Radical Biology and Medicine. Skin fibrosis in SSc tied to excess of fibroblasts and collagen SSc, also known as scleroderma, is an autoimmune disease wherein scar tissue accumulates in the skin and, in another disease type, in internal organs as well. Skin fibrosis is caused by the rapid and uncontrolled growth, or proliferation, of cells called fibroblasts and excessive production of collagen, a connective tissue protein. However, it is not clear what initially drives these processes, and current disease treatments aim to manage symptoms associated with scleroderma. No available therapies are specifically designed to target fibrosis in SSc, the study’s researchers stated. Programmed cell death refers to types of cell death that occur naturally and serve important biological functions. Ferroptosis is one form of programmed cell death that’s believed to help prevent the excessive cell growth that characterizes cancer. In ferroptosis, iron triggers the overproduction of toxic reactive oxygen species (ROS) molecules. This leads to a state of oxidative stress, an imbalance between ROS and the antioxidant molecules that counterbalance them, which ultimately drives cell death. As such, antioxidants can regulate ferroptosis. This form of programmed cell death has been identified as a possible therapeutic target in SSc, but the exact relationship remains to be thoroughly investigated. Updates shared at 2024 EULAR Congress may help 'better manage' SSc care by Marisa Wexler, MS | June 25, 2024 Treatment with Letairis (ambrisentan) — approved to treat pulmonary arterial hypertension (PAH), a common complication of systemic sclerosis (SSc) — may help prevent the development of PAH, or high blood pressure in the arteries of the lungs, in people with SSc.
That’s according to new data from SSc patients who completed the Phase 2 EDITA clinical trial (NCT02290613), which tested Letairis against a placebo, and who then continued their assigned regimen over a longer period. These results, along with positive data from the Phase 2 AST-MOMA trial (NCT01895244) — which evaluated a reduced-toxicity regimen of stem cell transplant in SSc patients — were presented at the 2024 European Alliance of Associations for Rheumatology (EULAR) Congress, held earlier this month in Vienna. “Taken together, these new findings could have an important bearing on the current standard of care for patients with SSc,” the alliance stated in a press release. Overall, EULAR stated that “new, strong evidence is now available to help better manage patients with this life-threatening condition,” noting, however, that “gaps remain.” Therapy is designed to block PAI-1, a mediator of inflammation, fibrosis by Patricia Inácio, PhD | June 18, 2024 The first healthy volunteers have been dosed in a Phase 1 clinical study that’s evaluating MDI-2517, a small molecule candidate to treat scleroderma and interstitial lung disease (ILD), which occurs when the lungs become scarred.
According to developers MDI Therapeutics, MDI-2517 is a potent blocker of plasminogen activator inhibitor-1 (PAI-1), a protein and key mediator of inflammation and fibrosis, or tissue scarring and thickening. “Based on comprehensive preclinical studies, this first in-human study of MDI-2517 will inform continued development of our novel, proprietary compound for the potential treatment of systemic sclerosis and interstitial lung disease, conditions where disability is significant and survival rates are poor,” Mark Weinberg, MD, chief medical officer at MDI Therapeutics, said in a company press release. In scleroderma, a self-reactive immune system causes fibrosis in the skin and possibly in internal organs, including the heart, kidney, lungs, and digestive tract. The disease is thought to develop due to a combination of genetic and environmental factors, resulting in the attack of body tissues by so-called autoantibodies. Study finds subclinical issues in 25% of patients using 'more sensitive' tool by Steve Bryson, PhD | June 11, 2024 Problems with the heart’s left ventricle at a subclinical — not yet symptomatic and overt — level were evident in 1 in every 4 people with systemic sclerosis (SSc) taking part in an Australian study when a potentially more sensitive measure than left ventricle ejection fraction was used.
Both symptomatic or asymptomatic left ventricle dysfunction also associated with poorer physical function and survival, data showed. These findings suggest “an under-appreciated burden of subtle LV [left ventricle] systolic dysfunction in SSc that has a significant impact on patient symptomatology,” the researchers wrote. The study, “Prognostic and functional importance of both overt and subclinical left ventricular systolic dysfunction in systemic sclerosis,” was published in the journal Seminars in Arthritis and Rheumatism. Left ventricle ejection fraction measures were normal in most study patients SSc, also known as scleroderma, is marked by the abnormal buildup of scar tissue (fibrosis) in the skin and, potentially, other organs like the lungs, kidneys, heart, and those of the digestive system. Heart involvement can be life-threatening in SSc, and advanced imaging techniques have shown scar tissue formation in heart muscle in up to 90% of patients without symptoms, the researchers noted. Asymptomatic myocarditis, or inflammation of the heart muscle, may also occur. Despite these abnormalities, little is known about scleroderma-related dysfunction of the left ventricle, the side of the heart that pumps oxygenated blood throughout the body. To address this gap, scientists across Australia collaborated to investigate the frequency of reduced left ventricle function in SSc. A total of 1,141 patients were recruited from the Australian Scleroderma Cohort Study, a multicenter and observational disease study. All had at least one measure taken of left ventricle ejection fraction (LVEF), the percentage of blood pumped from the left ventricle with each heartbeat. Patients with a history of secondary or non-SSc causes of left ventricle dysfunction were excluded. Nearly all participants (97.6 %) had a consistently normal LVEF of at least 50%, while 27 (2.4 %) had an LVEF of less than 50% at least once. Seven of them (0.6 %) had an LVEF of less than 40%. Those with an LVEF of less than 50% were more likely to be male, to have diffuse cutaneous SSc, a larger left atria (the top left heart chamber), higher peak blood pressure in the right ventricle, and more frequent right ventricular dysfunction. These 27 patients also were more likely to have higher levels of inflammatory markers and to more often use medicines for heart-related issues and immunosuppression. Diseases that affected skeletal muscle, those attached to bones, also were more frequent in patients with an LVEF of less than 50%. The self-reactive antibodies are an indicator of Sjögren’s syndrome by Patricia Inácio, PhD | May 21, 2024 Anti-SSA self-reactive antibodies, a hallmark of Sjögren’s syndrome, are associated with a higher risk of organ involvement in people with scleroderma, including the lungs and skin, a study in Japan shows.
The findings suggest “anti-SSA may be a valuable biomarker of organ involvement and skin severity in patients with SSc [systemic sclerosis, also called scleroderma],” wrote the researchers, who said “periodic screening for these involvements should be recommended in patients with SSc” who are positive for such antibodies. The study, “Clinical features of patients with systemic sclerosis positive for anti-SS-A antibody: a cohort study of 156 patients,” was published in Arthritis Research and Therapy. Sjögren’s syndrome is a chronic inflammatory autoimmune disease wherein a misdirected immune attack affects the glands that produce tears and saliva. It’s called primary Sjögren’s syndrome when it appears as an isolated illness and secondary when it follows another autoimmune disease. Anti-SSA antibodies are a diagnostic marker of Sjögren’s syndrome and are often detected in people with SSc. In fact, studies report that up to 24% of SSc patients have Sjögren’s syndrome. While SSc patients may have anti-SSA antibodies, but not symptoms of Sjögren’s syndrome, “few studies have focused on patients with anti-SSA-positive SSc, and the clinical significance of this presentation is not fully understood,” wrote researchers in Japan who analyzed data from 156 patients with SSc who were followed at the Yokohama City University Hospital between 2018-2021. The patients were mostly (88.5%) women, were a median age of 69 and a mean disease duration of 13.3 years. As for SSc subtypes, most (75%) had limited cutaneous SSc and 25% had diffuse cutaneous SSc. I share my story and hope it inspires someone to help in the fight by Sherlene Perkins | May 20, 2024 I love to have conversations with strangers and tell them about scleroderma.
I take time during brief encounters at the library, supermarket lines, the nail salon, and airport gates. The challenge is to provide the information in two minutes or less to spark and keep the listener’s attention. I ask if they are familiar with scleroderma and explain how it sneaks in and attacks parts of the body, causing arthritic pain, loss of fingers and toes, skin tightness, and the hardening of organs. I show them my hands and do the pinch test to demonstrate the thickness of my skin. I also provide some general terminology while telling them that many patients diagnosed with scleroderma experience skin tightening, Raynaud’s phenomenon, and gastroesophagael reflux disease (GERD). Some may also have lung fibrosis, interstitial lung disease, and pulmonary hypertension. I make a point to mention hip-hop singer and actress Queen Latifah, and explain that scleroderma is what her mom died from. The late actor Bob Saget lost his sister to scleroderma as well. During my chats, I mention the advocacy organizations I have been involved with that help rare disease patients maintain or improve their quality of life. These include the National Organization for Rare Disorders, Global Genes, the Rare Disease Legislative Advocates, the University of Michigan’s RENEW Scleroderma app, Northwestern Medicine, and Rare Patient Voice. I will even direct people to my website. I encourage them to get involved with helping those less fortunate and explain we need access to better healthcare and other supportive services. I tell them that scleroderma is complicated. At times, the disease is silent, and someone won’t know what’s going on until new symptoms appear or an old one reappears. Few vaccinated patients reported symptoms worsening, according to survey by Margarida Maia, PhD | June 4, 2024 More than half of people with systemic sclerosis (SSc) who hesitated to get vaccinated against COVID-19 were concerned they might have a flare-up, but very few vaccinated patients have reported their symptoms worsening, according to a 2021–2022 survey study.
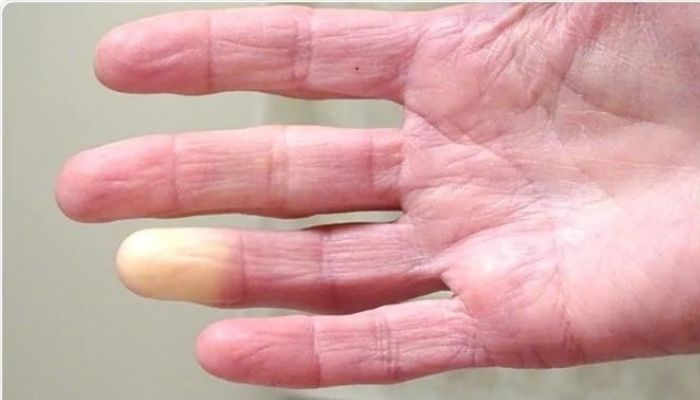
“Even though the peak of the pandemic has passed, COVID-19 infections and hospitalizations continue to present substantial risk for vulnerable people with autoimmune rheumatic diseases, including SSc,” said the study’s researchers, who noted that patients who are unsure about a vaccination should be informed of its safety. The study, “COVID-19 vaccinations and infections among individuals with systemic sclerosis: a Scleroderma Patient-centered Intervention Network (SPIN) Cohort study,” was published in Seminars in Arthritis and Rheumatism. When vaccines against COVID-19 were made available, there was little information on their safety in people with autoimmune rheumatic diseases. Concerns that getting one may have serious side effects led many people to hesitate. Treatment is ‘promising option,’ more studies needed, researchers say by Margarida Maia, PhD | May 14, 2024 Local injection with botulinum toxin type A (BTX-A), such as Botox, may ease the severity of pain associated with Raynaud’s phenomenon in people with scleroderma, also known as systemic sclerosis, a meta-analysis study found.
The study, “Efficacy and safety of botulinum toxin in treating scleroderma-associated raynaud’s phenomenon: a systematic review and meta-analysis,” was published as a research letter in the Archives of Dermatological Research. Scleroderma affects the connective tissue that holds tissues and organs together. While it mainly causes the skin to harden, scleroderma also can damage internal organs and blood vessels. It often starts with Raynaud’s phenomenon, which may appear long before other symptoms. Raynaud’s phenomenon limits blood flow to fingers and toes due to cold or stress. As a result, fingers and toes can change color and become numb or prickly. This is often accompanied by pain and sometimes digital ulcers or sores. Bacteria-derived BTX-A, often injected locally to smooth wrinkles on the face, among other uses, is being tested as a treatment for Raynaud’s phenomenon secondary to scleroderma. It’s thought that it may work in part by widening the blood vessels and reducing pain. Almost 20% of people in study have low red blood cells or low hemoglobin by Patricia Inácio, PhD | May 7, 2024 Anemia is found in almost 20% of people with systemic sclerosis (SSc) and is associated with an almost two times higher risk of death, a French study reports.
Iron deficiency was the most common reason for anemia in SSc, with older patients and those with diffuse cutaneous SSc (dcSSc) more likely to have anemia, which is a low number of red blood cells or low levels of hemoglobin. The study, “Prevalence, causes, and clinical associations of anemia in patients with systemic sclerosis: A cohort study,” was published in the Journal of Scleroderma and Related Disorders. Also called scleroderma, SSc typically leads to scarring in the skin due to the excessive production of collagen, the main protein component of scar tissue. Scarring can also affect internal organs, including the lungs, heart, kidney, and digestive tract. Anemia is considered a risk factor for more severe outcomes in SSc. Here, a team led by researchers in France analyzed data from 502 SSc patients (mean age, 58; 85.7% women), diagnosed at the Lille University Hospital to learn more about how frequently anemia occurs and the underlying causes of it in SSc. Most of the patients (77.5%) had limited SSc and 21.6% had dcSSc. |
AuthorScleroderma Queensland Support Group Archives
July 2024
Categories
All
|
Scleroderma Association of Queensland
©Scleroderma Association of Queensland. All rights reserved. Website by Grey and Grey.