|
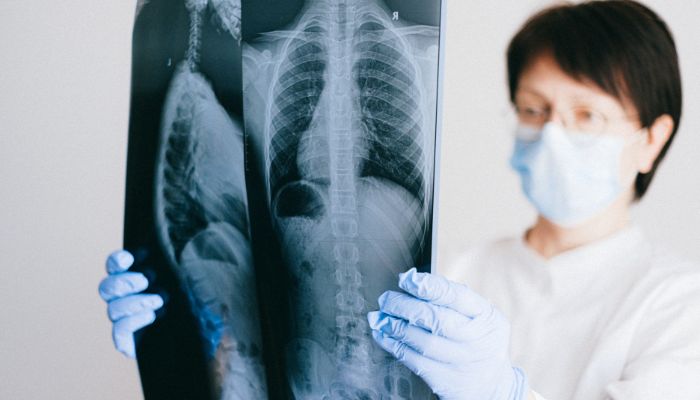
Oral therapy shown to safely improve lung function in Phase 2 trial by Andrea Lobo, PhD | February 20, 2024 Certa Therapeutics’ FT011, an investigational oral therapy for systemic sclerosis (SSc), has been granted fast track designation by the U.S. Food and Drug Administration (FDA). It comes following Phase 2 clinical trial data that showed that FT011 was safe and well tolerated, and led to clinically meaningful improvements in lung function for some participants. The therapy also helped to ease physical disability for more than half of the patients in the small study. The FDA fast track designation, which provides incentives such as more frequent meetings with the regulatory agency and potential eligibility for priority review and accelerated approval, is meant to speed the development of treatments for serious or life-threatening conditions. “We are thrilled to have received fast track designation which supports further acceleration of the FT011 clinical development program,” Darren Kelly, PhD, Certa’s founder and CEO, said in a company press release. Kelly added that the designation “also provides validation of FT011’s potential to offer patients with scleroderma the first anti-fibrotic and disease modifying treatment of this type.” Pivotal clinical trial of FT011 expected to launch later this year
Systemic sclerosis or SSc, also known as scleroderma, is an autoimmune disorder characterized by inflammation and the accumulation of scar tissue in the skin and potentially in several internal organs. FT011 is designed to block the activity of a protein receptor called GPR68, for G protein-coupled receptor 68, which is involved in driving scarring, or fibrosis, and has not been targeted by other medications. While GPR68 is normally silent, it is activated after injury or during diseases. According to the company, the therapy demonstrated efficacy in reverting the activation of genetic markers associated with fibrosis. It also was granted orphan drug status in the U.S. last fall — another FDA designation that gives therapies, if approved, a seven-year period of market exclusivity. “To date, existing treatments only focus on the relief and management of symptoms, whereas FT011 precisely targets the root cause of fibrosis and has the potential to offer treatment across multiple organs within these patients,” Kelly said. The Phase 2 trial (NCT04647890) enrolled 30 adults with diffuse cutaneous SSc, who were randomly assigned to either oral FT011 at 200 or 400 mg, or to a placebo. The treatment was given in addition to standard of care therapies for 12 weeks, or about three months. Overall, 60% of patients treated with high-dose FT011 and 20% of those receiving the lower dose experienced clinically meaningful improvements, compared with 10% of participants given the placebo. These improvements were assessed via American College of Rheumatology Combined Response Index in Systemic Sclerosis (ACR-CRISS) scores; the ACR-CRISS is a composite measure of disease severity and organ damage. To date, existing treatments only focus on the relief and management of symptoms, whereas FT011 precisely targets the root cause of fibrosis and has the potential to offer treatment across multiple organs within these patients. Patients receiving FT011 also experienced a reduction in skin thickness, and an improvement in forced vital capacity, a measure of lung function based on how much air a person can blow out in a forceful breath. Both patient- and physician-rated measures of health further tended to favor the therapy over the placebo. Moreover, no serious safety issues were reported, with overall rates of side effects similar for FT011 and the placebo. Some participants chose to continue treatment, receiving the 400 mg FT011 dose for nine more months. The company now is preparing a pivotal clinical trial of FT011 for scleroderma, aiming to start it later this year. Trial design and development plans will be discussed with the FDA in the U.S. and with the European Medicines Agency in Europe. Comments are closed.
|
AuthorScleroderma Queensland Support Group Archives
July 2024
Categories
All
|
Scleroderma Association of Queensland
©Scleroderma Association of Queensland. All rights reserved. Website by Grey and Grey.