|
Cabaletta Bio developing CAR T-cell therapy for other autoimmune conditions by Marisa Wexler, MS | January 9, 2024 The U.S. Food and Drug Administration (FDA) has granted fast track designation to the cell therapy CABA-201 for organ dysfunction in people with scleroderma. The agency also granted CABA-201 fast track status to reduce disease activity in people with dermatomyositis, another rare disorder that’s marked by muscle weakness and skin rash. The FDA had previously given CABA-201 fast track designation as a potential treatment for lupus. “The additional Fast Track Designations for CABA-201 … provide the opportunity for expedited development and review of CABA-201 for the treatment of these autoimmune indications where there is a significant unmet need, despite currently available therapies,” David Chang, MD, chief medical officer at Cabaletta Bio, which is developing CABA-201, said in a company press release. A fast track designation is given to speed up the development of therapies that have the potential to address unmet medical needs for serious conditions. The designation provides Cabaletta with incentives including more frequent meetings with the FDA during the therapy development process, in addition to potential eligibility for accelerated approval.
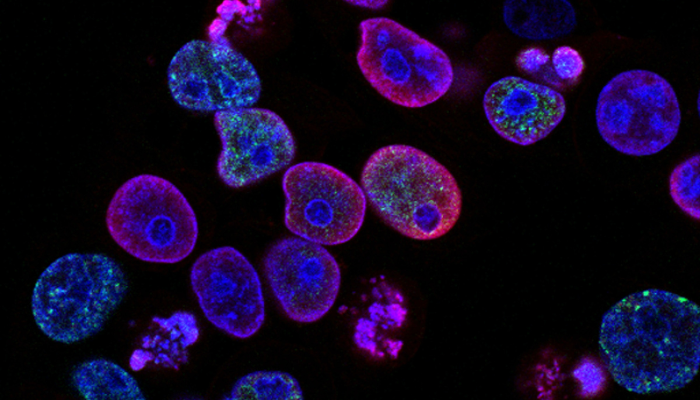
“We believe these designations potentially accelerate our ability to launch the first targeted, and potentially curative, cell therapy for autoimmune diseases driven by B cells,” Chang said. “We look forward to continuing to leverage our research and translational insights along with our efficient trial designs in order to progress these programs forward for patients in need of better outcomes.” CABA-201 is CAR T-cell therapy designed to deplete B-cells CABA-201 is a CAR T-cell therapy designed to deplete B-cells, a type of immune cell that plays a central role in the autoimmune attack that drives scleroderma (also known as systemic sclerosis). The therapy works by harvesting T-cells from a patient, then engineering the cells to equip them with a human-made protein called a chimeric antigen receptor or CAR. The T-cells specifically are equipped with a CAR that directs the cells to attack CD19, a protein that’s expressed by B-cells. The modified T-cells are then infused back into the patient, with the goal of destroying B-cells for durable disease remission. The announcement of fast track designation for scleroderma comes a few months after the FDA cleared Cabaletta to launch a Phase 1/2 clinical trial that seeks to test the therapy in a dozen patients, including six with severe skin symptoms and another six with organ involvement regardless of skin manifestations. Comments are closed.
|
AuthorScleroderma Queensland Support Group Archives
July 2024
Categories
All
|
Scleroderma Association of Queensland
©Scleroderma Association of Queensland. All rights reserved. Website by Grey and Grey.